Biotech Stock Roundup: KYMR, IDYA, QURE Soar on Study Data, HLVX Tanks on Study Failure
It was a regular week for the biotech sector with quite a few important pipeline and regulatory updates. IDEAYA Biosciences IDYA and uniQure N.V. QURE were in the spotlight following positive study data from key studies.
Recap of the Week’s Most Important Stories:
KYMR Soars on Study Data: Kymera Therapeutics, Inc. KYMR announced that partner Sanofi SNY intends to rapidly expand the ongoing mid-stage studies on KT-474 (SAR444656) toward pivotal studies. KT-474 (SAR444656) is a first-in-class IRAK4 degrader in development for the treatment of immune-inflammatory diseases, such as hidradenitis suppurativa (HS) and atopic dermatitis (AD), with significant patient need. The candidate is in phase II studies for HS and AD.
Sanofi informed Kymera about the decision to expand the study after a review of preliminary safety and efficacy data in these studies by an Independent Data Review Committee. Sanofi has collaborated with Kymera on the development of KT-474 outside the oncology and immuno-oncology fields. The pipeline progress impressed investors and shares of KYMR gained on the same. Kymera is deploying targeted protein degradation to address disease targets and pathways inaccessible with conventional therapeutics.
IDEAYA Up on Study Data: The company announced positive clinical data from its mid-stage study evaluating the 30 mg monotherapy expansion dose of IDE397 to treat heavily pre-treated methylthioadenosine phosphorylase (MTAP)-deletion urothelial (bladder) and non-small cell lung cancer (NSCLC) patients. The phase II clinical data for IDE397, based on 18 evaluable patients with MTAP deletion, includes seven with urothelial cancer, four with adenocarcinoma NSCLC and seven with squamous NSCLC. These patients were treated with the 30 mg expansion dose once a day. The reported clinical efficacy and tolerability data are preliminary, drawn from an investigator-reviewed unlocked database as of the cutoff date of Jun 21, 2024.
Per the data readout, the study achieved an overall response rate of 39%. Among the 18 evaluable patients, there was one complete response and six partial responses, according to the RECIST 1.1 evaluation. Two partial responses are pending confirmation, including a urothelial cancer patient with 100% tumor reduction and an adenocarcinoma NSCLC patient. The complete response and two partial responses were in urothelial cancer patients. Three partial responses were observed in squamous NSCLC patients and one in an adenocarcinoma NSCLC patient. One patient discontinued due to rapid disease progression and drug-unrelated adverse events.
A disease control rate of 94% was also observed in the study. Furthermore, 14 out of 18 evaluable patients (78%) experienced tumor shrinkage. Additionally, 13 out of 16 patients, who represented 81% of the evaluable patient population, experienced a ctDNA reduction of 50% or more. The IDE397 expansion dose of 30 mg achieved target drug coverage and plasma S-adenosyl-l-methionine pharmacodynamic reduction associated with preclinical tumor regressions. Shares of IDYA gained on the results.
uniQure Soars on Study Data: The company announced updated interim data, including up to 24 months of follow-up data, from 29 treated patients enrolled in the ongoing early to mid-stage studies of gene therapy AMT-130 for Huntington’s disease in the United States and EU.
uniQure is simultaneously conducting two phase I/II studies of AMT-130, with 26 participants in the United States and 13 in the EU/UK, to treat Huntington's disease. Among these, a total of 29 patients received either a low dose (12 patients) or a high dose (17 patients) of AMT-130, while 10 control patients underwent imitation surgery. As of Mar 31, 2024, follow-up data at 24 months were available for 21 patients, including 12 from the low-dose group and nine from the high-dose group.
Per the data readout from the phase I/II Huntington’s disease studies, patients treated with the high dose of AMT-130 experienced a statistically significant, dose-dependent slowing in disease progression of 80%, as measured by cUHDRS after 24 months. For the low-dose group, a 30% slowing of disease progression, as measured by cUHDRS, was observed compared with the control group at month 24.
Results demonstrated a potential long-term, durable clinical benefit and reduction of a key marker of neurodegeneration upon treatment with the candidate. The company is currently gearing up to hold a meeting with the FDA in the second half of 2024 to discuss these updated positive data seeking potential expedited clinical development pathways and accelerated approval for AMT-130.
uniQure currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
HilleVax Down on Study Failure: HilleVax, Inc. HLVX announced that its phase IIb NEST-IN1 study, evaluating its investigational virus-like particle-based vaccine candidate, HIL-214, in infants for the prevention of acute gastroenteritis (AGE) caused by norovirus infection, failed to meet its primary or secondary efficacy endpoints.
The double-blind, placebo-controlled study evaluated the efficacy, safety and immunogenicity of HIL-214 in infants around five months old.
Per the company, the NEST-IN1 study had 51 primary endpoint events, with 25 in the vaccine arm and 26 in the placebo arm. Data from the study showed that HIL-214 had a vaccine efficacy of only 5% against moderate or severe norovirus-related AGE.
The NEST-IN1 study failed to meet its primary efficacy endpoint against moderate or severe AGE events related to GI.1 or GII.4 norovirus genotypes. Also, vaccination with HIL-214 failed to demonstrate a clinical benefit across the study’s secondary endpoints. Following the disappointing results, HilleVax decided that it would stop further development of HIL-214 in infants. Shares of HLVX tanked on the news.
Performance
The Nasdaq Biotechnology Index has gained 4.66% in the past four trading sessions, while Incyte’s shares have risen 5.32%. In the past six months, shares of REGN have rallied 18.20%. (See the last biotech stock roundup here: Biotech Stock Roundup: GSK, CVAC Revise Agreement, Updates From MRNA, RNAC & More)
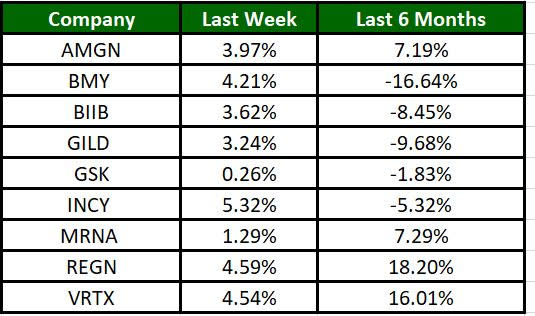
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for more pipeline updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
uniQure N.V. (QURE) : Free Stock Analysis Report
IDEAYA Biosciences, Inc. (IDYA) : Free Stock Analysis Report
Kymera Therapeutics, Inc. (KYMR) : Free Stock Analysis Report
HilleVax, Inc. (HLVX) : Free Stock Analysis Report