Coronavirus update: All 50 states lift some restrictions as questions surface over Moderna data
More economic re-openings continued on Wednesday — with all 50 U.S. states seeing some degree of lockdown measures being relaxed this week — even as global coronavirus diagnoses and death tolls mounted.
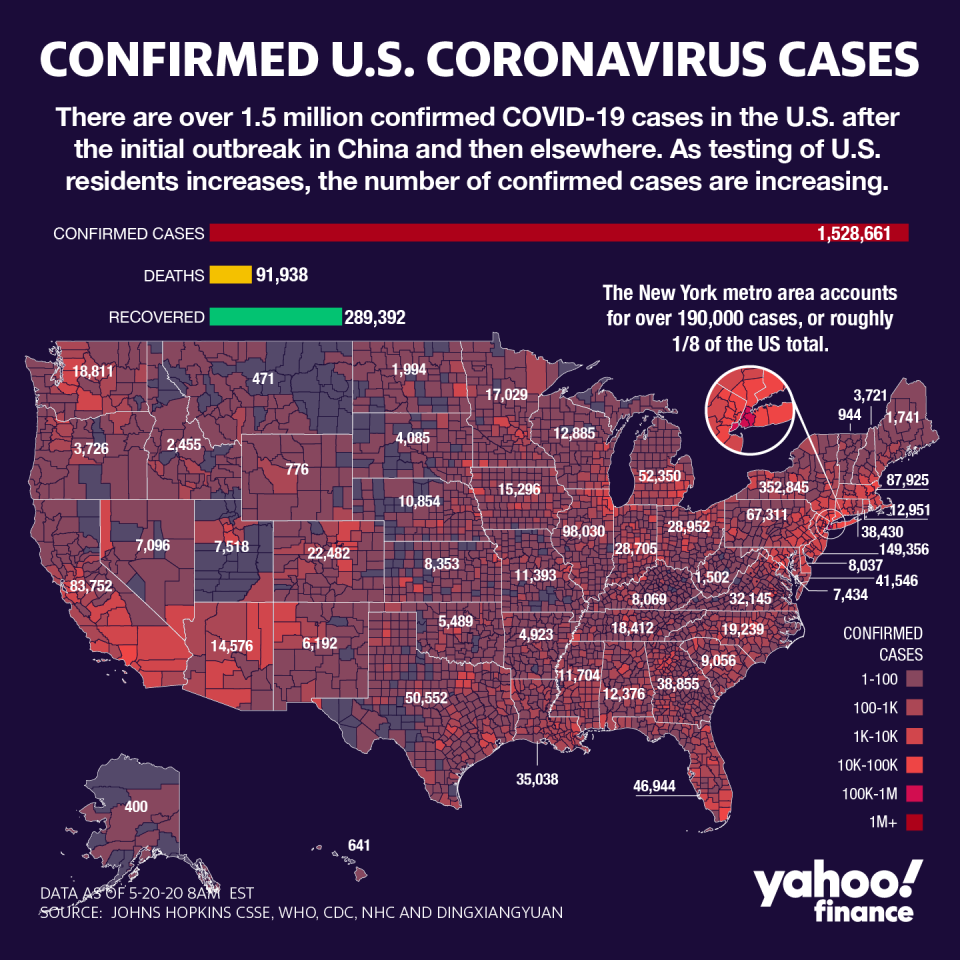
The U.S. has seen a significant slowing of the virus spread in most states, and previous major hotspots are showing consistent day-over-day declines — including New York, where Gov. Andrew Cuomo announced more measures designed to ease the restrictions imposed on the U.S.’s hottest COVID-19 zone. Antibody testing data showed that the Big Apple had a positive rate of nearly 20%.
Nevertheless, the global case count is creeping toward 5 million, with more than 320,000 dead. In the U.S. more than 1.5 million are infected and more than 93,000 have died.
Amid a politically charged war of words with the United States, the World Health Organization warned that President Donald Trump’s threat to yank funding over complaints that the organization was too close to China would damage the agency’s efforts to help the poor.
Meanwhile, some large employers, like tech giants and financial services firms, are maintaining work-from-home policies until the end of 2020.
Meanwhile, others are focused on return to work strategies and the elements needed to do so, like questionnaires, temperature scans and on-site testing. The Labor Department plans to expand inspection criteria to include coronavirus-related hazards, as some employees begin heading back to the office.
Schools are also faced with the same dilemma of reopening, with uneven results. In the U.S., California’s public universities have chosen to stay remote for the upcoming school year. In the U.K., meanwhile, Cambridge University has become the first to announce remote schooling for 2020-2021.
Curbing Moderna’s enthusiasm
Moderna (MRNA) has been under the spotlight this week, after announcing its experimental vaccine had produced positive results in a Phase 1 trial. The market has been largely underpinned by the results, but investors took a breather after a STAT report highlighted reasons to be cautious, as some health experts have articulated.
The actual data has not yet been released — which according to Criag Garthwaite, director of the program on healthcare at Northwestern University’s Kellogg School of Management, is the crux of the problem.
“It was a discussion of [just] a portion of a set of Phase 1 clinical trial results,” Garthwaite said.
However, Moderna’s CEO Stephane Bancel told Fox Business in an interview that the company was clear the information was “interim data.”
The scrutiny surrounding Moderna mirrored that on Gilead Sciences (GILD), when it released its company and National Institute of Health sponsored clinical trial results for an antiviral treatment. Gilead’s stock swung wildly in the days following its data release.
Yet health experts say there is still some reasons to be optimistic about Moderna’s treatment, given that the virus has shown to be impacted, even if minimally, in both rodent and animal trials. Trial information released later down the road will be more informative, and can provide greater certainty about the vaccine.
Testing ramps up
Among public health officials, contact testing is a hot-button issue in the fight against the coronavirus pandemic, and the move to reopen states. As a result, approximately 23 countries are seeking access to new technology from Apple (AAPL) and Alphabet Inc's Google (GOOG), which uses mobile apps to log users who are in physical proximity for at least five minutes.
Meanwhile, amid questions about who pays for COVID-related tests, the Centers for Medicare and Medicaid Services (CMS) released its reimbursement rate for antibody testing. The reimbursement rate of $42.13 sheds some light of the ballpark of insurer reimbursement for labs who process the test, but it is yet unclear if all antibody testing will be covered for patients.
The CMS rate was lauded by the American Clinical Laboratory Association (ACLA) which has previously been calling for higher reimbursements to support the costs of the sudden demand on labs nationwide to process unprecedented volumes of tests.
Separately, as the U.S. COVID casualties level off, U.S. Food and Drug Administration (FDA) released guidance saying that converted freezer trucks — which have been used to store the overwhelming number of dead patients near hospitals in some of the worst hit areas — can haul food again.
Anjalee Khemlani is a reporter at Yahoo Finance. Follow her on Twitter: @AnjKhem
[Click here for more of Yahoo Finance’s coronavirus coverage: Personal finance tips, news, policy, graphics & more from Yahoo Finance]
Follow Yahoo Finance on Twitter, Facebook, Instagram, Flipboard, LinkedIn, and reddit.
Find live stock market quotes and the latest business and finance news.

 Yahoo Finance
Yahoo Finance 